Details of the Drug
General Information of Drug (ID: DMC8J6F)
| Drug Name |
METHYLTHIOADENOSINE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
5'-Deoxy-5'-methylthioadenosine; 2457-80-9; Methylthioadenosine; 5'-S-methyl-5'-thioadenosine; 5'-Deoxy-5'-(methylthio)adenosine; 5'-DEOXY-5'-METHYLTHIOADENOSINE; (2R,3R,4S,5S)-2-(6-Amino-9H-purin-9-yl)-5-((methylthio)methyl)tetrahydrofuran-3,4-diol; Thiomethyladenosine; Vitamin L2; ADENOSINE, 5'-S-METHYL-5'-THIO-; 5'-S-Methylthioadenosine; MTA; S-methyl-5'-thioadenosine; Vitamin L(sub 2); UNII-634Z2VK3UQ; 5'-Deoxy(methylthio)adenosine; 5'-Deoxy-5'-Methylthioadenosine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
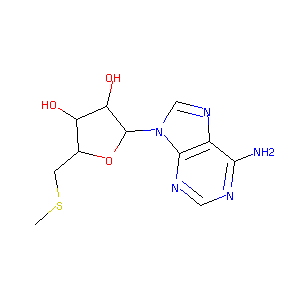 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 297.34 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| ICD Disease Classification | 08 Nervous system disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Class | ICD-11: 8A40 Multiple sclerosis | |||||||||||||||||||||||
| The Studied Tissue | Plasmacytoid dendritic cells | |||||||||||||||||||||||
| The Studied Disease | Multiple sclerosis [ICD-11:8A40] | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
